Silica Versus Pandemic
The coronavirus pandemic has drawn the attention of companies from across the private sector, all seeking to use their expertise to curtail the severity of the crisis. Grace was there to lend a hand, providing silica products for tests that detect SARS-CoV-2, the virus that causes COVID-19; for purification of key vaccine adjuvants; and for formulating drugs that treat COVID-19.
A critical test
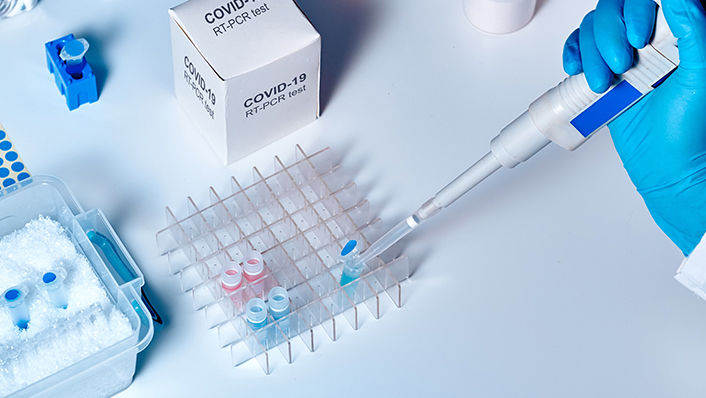
One of the most common tests that public health authorities use to monitor the spread of SARS-CoV-2 is a reverse transcriptase PCR (RT-PCR) test, in which a swab sample collected from a person’s nose or throat is tested for viral RNA. To isolate those nucleic acids from other biomolecules that would confound the test results—such as lipopolysaccharides from bacteria that live in the nose and mouth or proteins from the human body's own cells—scientists use DAVISIL® XWP silica, a product from Grace.
Introduced in the late 1990s for the purification of biological molecules, DAVISIL® XWP silica has extra-wide pores that allow large biomolecules to permeate the silica gel. The biomolecules can then interact with silanol groups on the surface of the silica gel to facilitate separation.
The isolation of viral RNA begins by treating the swab sample with a buffered solution containing a high concentration of salts. Reagents in the buffer solution disrupt the shell of water molecules that surround nucleic acids, exposing the negatively-charged phosphate groups of the nucleic acid backbone. Thus exposed, the phosphate groups attract positively-charged salt ions, which in turn are attracted to the silanol groups of the silica gel support, that are negatively charged under the buffer conditions. The resulting sandwich-like arrangement causes nucleic acids to cling to the silica gel while other, extraneous biomolecules are washed away. The purified nucleic acids can then be subjected to amplification by RT-PCR and sequencing to confirm whether the unique "fingerprint" of SARS-CoV-2 is present.
While all silica particles possess the silanol groups that enable this separation, they are not the only factor at play. The efficiency of the method owes a great deal to the physical characteristics of the silica particles—that is, their surface area and porosity, which in turn are affected by the size of the particles and their pores. DAVISIL® XWP silica turns out to be particularly well-suited to SARS-CoV-2 detection, and is available in a wide range of pore sizes from 500–4500Å, allowing scientists to fine-tune their purification strategy.
Scalable, compendial silicon dioxide, meeting monograph requirements for several national pharmacopeias
Prinicpal Scientist
Excipients for extraordinary times
Grace's silica products also have applications in the formulation of drugs to potentially prevent and treat COVID-19, among other diseases. The company's SYLOID® line of silica can be used as a multifunctional excipient to manufacture solid dosage forms. It can also be mixed with drug substances isolated as a liquid or oil, allowing scientists to dose these materials as a more manageable solid power. The large surface area of the SYLOID® silica particles allows scientists to fit a surprising amount of the active ingredient into a relatively small capsule or tablet.
Reno Nguyen, principal scientist at Grace, describes the company’s offerings in this area as scalable, compendial silicon dioxide that meets monograph requirements for several national pharmacopeias and enables them to be used in commercial API processing. That's an important attribute in a year when medical research has taken place with unusual urgency and speed.
A race to vaccinate the world
DAVISIL® silica is a proven technology that has been used in good manufacturing practice (GMP) vaccine manufacturing for well over a decade. Grace’s DAVISIL® silica gel is a part of the purification process for some key COVID-19 vaccine adjuvants, owing to the product’s outstanding lot-to-lot reproducibility and high loading capacity. With the wide range of pore size offerings, DAVISIL® silica may be used in purifying lipids, nucleic acids, proteins, and virus-like particles (VLP)—all of which may potentially play a role in ending the COVID-19 pandemic.